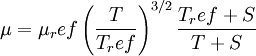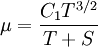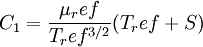Sutherland's law
From CFD-Wiki
(Difference between revisions)
| Line 1: | Line 1: | ||
In 1893 [http://en.wikipedia.org/wiki/William_Sutherland_(physicist) William Sutherland], an Australian physicist, published a relationship between the dynamic visocity, <math>\mu</math>, and the absolute temperature, <math>T</math>, of an ideal gas. This formula, often called Sutherland's law, is based on kinetic theory of ideal gases and an idealized intermolecular-force potential. Sutherland's law is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures. Sutherland's law can be expressed as: | In 1893 [http://en.wikipedia.org/wiki/William_Sutherland_(physicist) William Sutherland], an Australian physicist, published a relationship between the dynamic visocity, <math>\mu</math>, and the absolute temperature, <math>T</math>, of an ideal gas. This formula, often called Sutherland's law, is based on kinetic theory of ideal gases and an idealized intermolecular-force potential. Sutherland's law is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures. Sutherland's law can be expressed as: | ||
| - | :<math>\mu = \ | + | :<math>\mu = \mu_ref \left( \frac{T}{T_ref} \right)^{3/2}\frac{T_ref + S}{T + S}</math> |
| - | :<math> | + | :<math>T_ref</math> is a reference temperature. |
| - | :<math>\ | + | :<math>\mu_ref</math> is the viscosity at the <math>T_ref</math> reference temperature |
:S is the Sutherland temperature | :S is the Sutherland temperature | ||
| Line 13: | Line 13: | ||
Comparing the formulas above the <math>C_1</math> constant can be written as: | Comparing the formulas above the <math>C_1</math> constant can be written as: | ||
| - | :<math>C_1 = \frac{\ | + | :<math>C_1 = \frac{\mu_ref}{T_ref^{3/2}}(T_ref + S)</math> |
{| align=center border=1 | {| align=center border=1 | ||
|+ Sutherland's law coefficients | |+ Sutherland's law coefficients | ||
| - | ! Gas !! <math>\mu_0 [\frac{kg}{m s}]</math> !! <math>T_0 [K]</math> !! <math>S [K]</math> !! <math>C_1 [\frac{kg}{m s | + | ! Gas !! <math>\mu_0 [\frac{kg}{m s}]</math> !! <math>T_0 [K]</math> !! <math>S [K]</math> !! <math>C_1 [\frac{kg}{m s K ^ {0.5}}]</math> |
|- | |- | ||
| Air | | Air | ||
| - | | <math> | + | | <math>1.716 \times 10^{-5}</math> |
| - | | | + | | <math>273.15</math> |
| - | | | + | | <math>110.4</math> |
| - | | | + | | |
|} | |} | ||
Revision as of 17:14, 17 May 2007
In 1893 William Sutherland, an Australian physicist, published a relationship between the dynamic visocity, 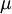 , and the absolute temperature,
, and the absolute temperature, 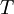 , of an ideal gas. This formula, often called Sutherland's law, is based on kinetic theory of ideal gases and an idealized intermolecular-force potential. Sutherland's law is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures. Sutherland's law can be expressed as:
, of an ideal gas. This formula, often called Sutherland's law, is based on kinetic theory of ideal gases and an idealized intermolecular-force potential. Sutherland's law is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures. Sutherland's law can be expressed as:
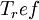 is a reference temperature.
is a reference temperature.
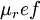 is the viscosity at the
is the viscosity at the 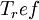 reference temperature
reference temperature
- S is the Sutherland temperature
Some authors instead express Sutherland's law in the following form:
Comparing the formulas above the 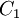 constant can be written as:
constant can be written as:
| Gas | ![\mu_0 [\frac{kg}{m s}]](/W/images/math/2/4/8/2480f45fd1c786230d032f54d5a4611f.png) | ![T_0 [K]](/W/images/math/1/e/6/1e63651a7e3249da3521fb3d1523c542.png) | ![S [K]](/W/images/math/e/4/4/e444c70ca8ff28fe00474e6ee27b3872.png) | ![C_1 [\frac{kg}{m s K ^ {0.5}}]](/W/images/math/3/0/5/3057483e72520517c1986e1b89cbc6aa.png)
|
|---|---|---|---|---|
| Air | 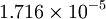
| 
| 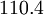
|
References
- Sutherland, W. (1893), "The viscosity of gases and molecular force", Philosophical Magazine, S. 5, 36, pp. 507-531 (1893).