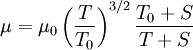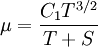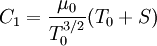Sutherland's law
From CFD-Wiki
(Difference between revisions)
(New page: * {{reference-paper|author=Sutherland, W.|year=1893|title=The viscosity of gases and molecular force|rest=Philosophical Magazine, S. 5, 36, pp. 507-531 (1893)}}) |
|||
| Line 1: | Line 1: | ||
| + | In 1893 [http://en.wikipedia.org/wiki/William_Sutherland_(physicist) William Sutherland], an Australian physicist, published a relationship between the absolute temperature, <math>T</math>, of an ideal gas and its dynamic visocity, <math>\mu</math>, based on the kinetic theory of ideal gases and an idealized intermolecular-force potential. This formula, often called Sutherland's law, is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures (up to several thousand degrees depending on the gas). Sutherland's law can be expressed as: | ||
| + | |||
| + | :<math>\mu = \mu_0 \left( \frac{T}{T_0} \right)^{3/2}\frac{T_0 + S}{T + S}</math> | ||
| + | |||
| + | Some authors instead express Sutherland's law in the following form: | ||
| + | |||
| + | :<math>\mu = \frac{C_1 T^{3/2}}{T + S}</math> | ||
| + | |||
| + | Comparing the formulas above the <math>C_1</math> constant can be written as: | ||
| + | |||
| + | :<math>C_1 = \frac{\mu_0}{T_0^{3/2}}(T_0 + S)</math> | ||
| + | |||
| + | == References == | ||
| + | |||
* {{reference-paper|author=Sutherland, W.|year=1893|title=The viscosity of gases and molecular force|rest=Philosophical Magazine, S. 5, 36, pp. 507-531 (1893)}} | * {{reference-paper|author=Sutherland, W.|year=1893|title=The viscosity of gases and molecular force|rest=Philosophical Magazine, S. 5, 36, pp. 507-531 (1893)}} | ||
Revision as of 13:38, 17 May 2007
In 1893 William Sutherland, an Australian physicist, published a relationship between the absolute temperature, 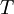 , of an ideal gas and its dynamic visocity,
, of an ideal gas and its dynamic visocity, 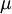 , based on the kinetic theory of ideal gases and an idealized intermolecular-force potential. This formula, often called Sutherland's law, is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures (up to several thousand degrees depending on the gas). Sutherland's law can be expressed as:
, based on the kinetic theory of ideal gases and an idealized intermolecular-force potential. This formula, often called Sutherland's law, is still commonly used and most often gives fairly accurate results with an error less than a few percent over a wide range of temperatures (up to several thousand degrees depending on the gas). Sutherland's law can be expressed as:
Some authors instead express Sutherland's law in the following form:
Comparing the formulas above the 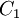 constant can be written as:
constant can be written as:
References
- Sutherland, W. (1893), "The viscosity of gases and molecular force", Philosophical Magazine, S. 5, 36, pp. 507-531 (1893).